Immunity | Daichao Xu’s Lab discovered that oxidized left-handed DNA, a new ligand of ZBP1, drives neuroinflammation in Alzheimer's disease
Date:2025-09-08
Alzheimer's disease (AD) is a kind of neurodegenerative disorder that remains uncurable, which is characterized by amyloid-β (Aβ) plaques and tau neurofibrillary tangles. With the intensification of aging in China, the prevalence of AD continues to rise, imposing a heavy economic burden on society and families. However, the development of AD therapeutics has remained notably slow.
Recent studies have demonstrated that microglia-mediated neuroinflammation plays a pivotal role in the pathogenesis and progression of AD. In AD, microglia are excessively and persistently activated by Aβ, thereby inducing chronic neuroinflammation, exacerbating tau pathology, promoting astrocyte dysfunction, and ultimately contributing to neuronal damage and degeneration. Multiple signaling pathways such as type I interferon, TLR signaling, and NLRP3 inflammasome have been reported to be involved in driving neuroinflammation, but the functional connections among them remain unclear.
Left-handed nucleic acid Z-DNA represents a non-canonical DNA secondary structure, which plays a pivotal role in innate immune activation through the Z-DNA-binding protein 1 (ZBP1) mediated signaling pathway. The identification of left-handed nucleic acid ligands has emerged as a pivotal scientific issue in this field. Additionally, studies conducted three decades ago revealed the presence of left-handed Z-DNA in AD patient brains, yet the underlying formation mechanisms, pathological roles, and the functional significance of ZBP1-mediated recognition remain entirely unexplored.
On September 2, 2025, the professor Daichao Xu’s Lab from the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, published a research paper called "Innate immune sensing of Z-nucleic acids by ZBP1-RIPK1 axis drives neuroinflammation in Alzheimer's disease" in Immunity. This article revealed that oxidatively fragmented Z-form mitochondrial DNA (Z-mtDNA) acts as a novel endogenous ligand for ZBP1, which drives the neuroinflammatory process mediated by microglia in AD. This discovery provides a new perspective for understanding the sensing of left-handed nucleic acids and the regulatory mechanism of neuroimmune in AD.

Xu’s lab discovered that in the pathological environment of AD, Aβ induced mitochondrial reactive oxygen species (mtROS) in microglia oxidized mtDNA guanine oxidizaition to generate 8-oxoG DNA. Subsequently, oxidized mtDNA was fragmented by mitochondria nucleases and released into the cytoplasm through the mPTP-VDAC channel. These 8-oxoG-containing mtDNA fragments undergo conformational changes to form Z-DNA, which can be specifically recognized by ZBP1 to mediate RIPK1 recruitment and subsequently kinase activation. This process simultaneously promotes multiple pro-inflammatory pathways such as type I interferons, TLR signaling, and NLRP3 inflammasome (Figure 1), ultimately leading to neuroinflammation and AD pathology.
Furthermore, ZBP1 deficiency or RIPK1 kinase inactivation in AD model significantly alleviates neuroinflammation, Aβ deposition, and behavioral defects. This result not only established the core role of the Z-DNA-ZBP1-RIPK1 axis in AD neuroinflammation, but also first clarified that endogenous Z-DNA produced by ROS serves as the key molecule activating this pathway.
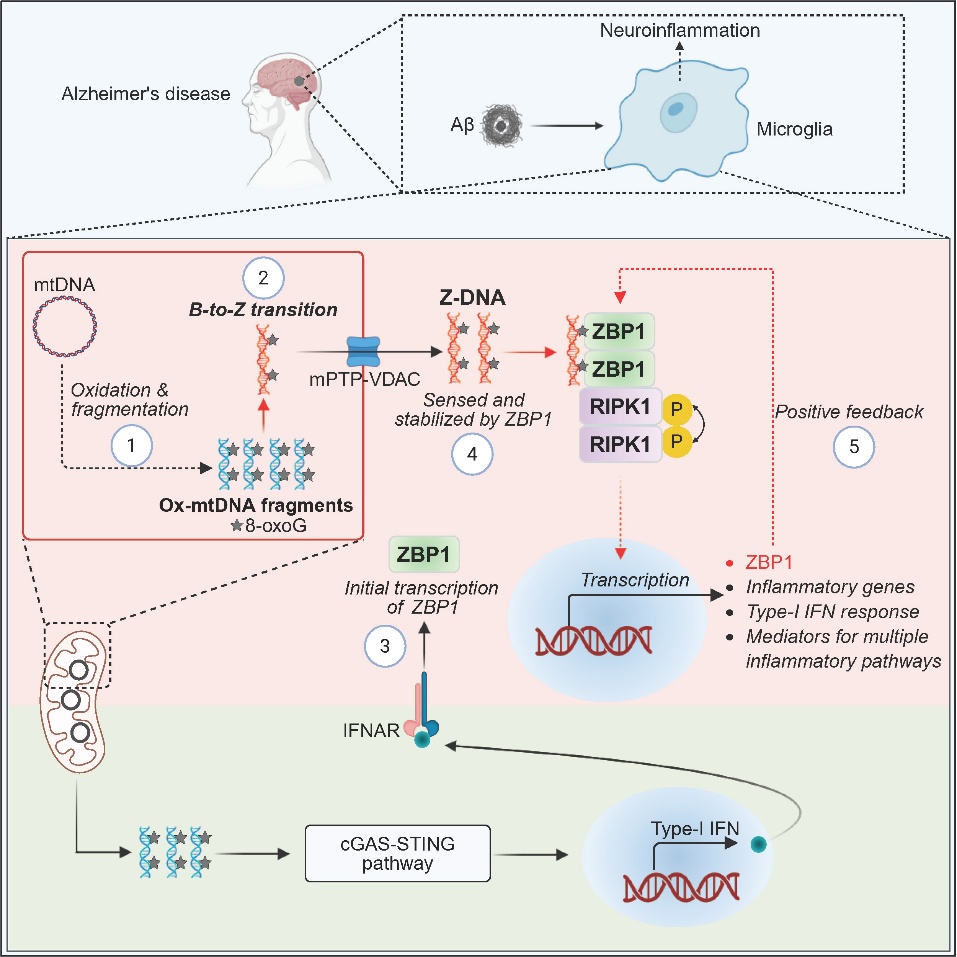
Figure 1. Generation of the novel oxidized left-handed DNA and its mechanism model in AD
In summary, this study identified oxidized left-handed DNA as a new ligand for ZBP1, breaking the previous notion that ZBP1 mainly recognizes exogenous pathogen nucleic acids or unmodified endogenous nucleic acids, which also provided a new perspective on the immune mechanism of AD. Since ROS is widely present in various human diseases, this discovery also provides important insights for the mechanism research and treatment strategies of other ROS-related human diseases. RIPK1 inhibitors are in the clinical trial stage currently. This study provides a theoretical basis for their application in AD treatment. In the future, RIPK1 inhibitors combined with Aβ-targeted therapy may be a new strategy for AD patients.
Professor Daichao Xu from the Interdisciplinary Research Center on Biology and Chemistry of the Chinese Academy of Sciences is the corresponding author of this paper. Professor Wei Mo from the Liangzhu Laboratory of Zhejiang University and Associate Professor Jian Zhang from Soochow University are the co-corresponding authors. Ziwen Song, a doctoral student from the Interdisciplinary Research Center on Biology and Chemistry of the Chinese Academy of Sciences, is the first author of this paper. Postdoctor Xingxing Xie and doctoral student Yulu Chen are the co-first authors.
附件下载:
