PNAS | A New Mechanism of PARP12 in Regulating Cell Death and Antiviral Immunity
Date:2025-06-13
Programmed cell death serves as a critical defense mechanism during viral infection. However, the molecular mechanisms governing virus-induced cell death and inflammatory responses remain incompletely understood. The kinases RIPK1 and RIPK3, central regulators of programmed cell death pathways, undergo precise modulation through various post-translational modifications. While ADP-ribosylation of RIPK1/3 has been documented, the functional consequences of this modification on kinase activity and downstream cell death signaling remain elusive. PARP family proteins are ADP-ribosyltransferases that can modify target proteins with ADP-ribose. As key ADP-ribosyltransferases, PARP family proteins have recently emerged as important regulators of antiviral immunity.
In a groundbreaking study published in PNAS (June 2025), the research team led by Professor Junying Yuan at the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, CAS, elucidated a novel regulatory mechanism mediated by PARP12. Their study, entitled "PARP12-mediated mono-ADP-ribosylation as a checkpoint for necroptosis and apoptosis," reveals how this mono-ADP-ribosyltransferase modulates cell fate decisions during viral infection.
Through systematic analysis of mass spectrometry data, the researchers identified PARP12 as a novel regulator of cell death pathways. Their mechanistic studies demonstrated that PARP12 specifically mono-ADP-ribosylates (MARylates) RIPK1 (targeting both intermediate and kinase domains) and RIPK3, thereby promoting RIPK1-RIPK3-dependent necroptosis while simultaneously suppressing RIPK1-caspase-8-mediated apoptosis. Furthermore, PARP12 was found to negatively regulate interferon-stimulated gene expression (including ZBP1) in a RIPK1-dependent manner.
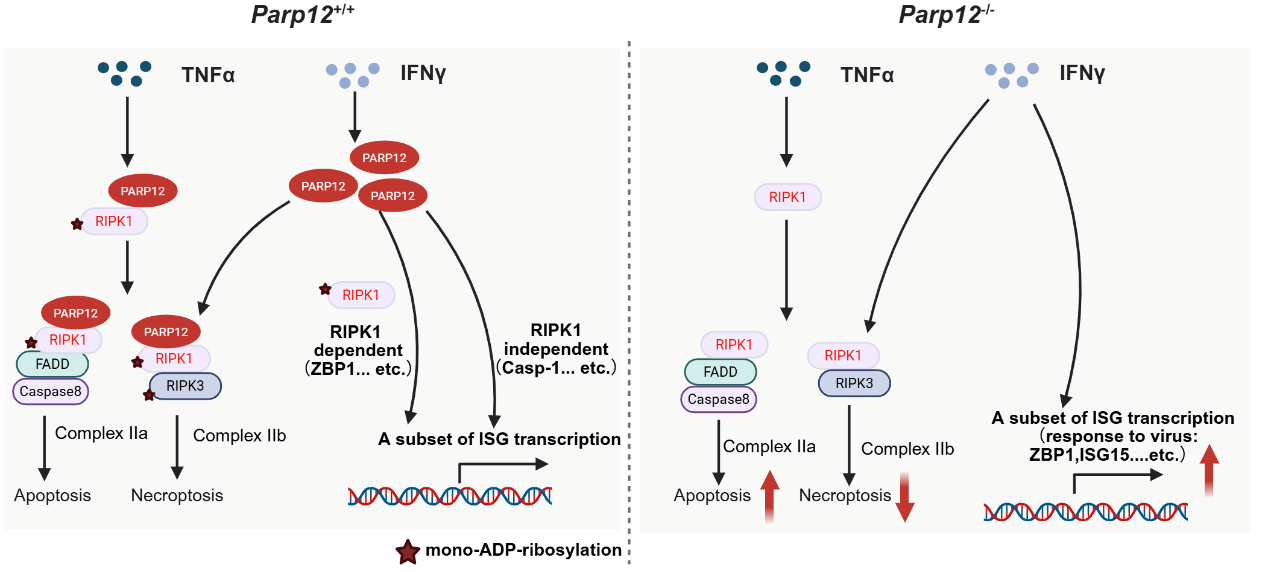
Fig.Schematic representation of how PARP12 regulates apoptosis, necroptosis, and interferon responses. (Image by Junying Yuan's group)
The physiological relevance of these findings was confirmed in vivo using PARP12-deficient mice. Following influenza virus infection, PARP12 knockout mice exhibited significantly improved survival outcomes and attenuated weight loss compared to wild-type controls. Histopathological examination revealed reduced pulmonary necroptosis and lower viral titers in PARP12-deficient animals, establishing PARP12 as a critical molecular switch governing cell death and immune responses during viral infection.
This work provides several important advances: (1) it identifies PARP12 as a novel regulator of cell death pathways, (2) elucidates a previously unknown mechanism of RIPK1/3 regulation through MARylation, and (3) reveals a molecular checkpoint that coordinates cell death and antiviral immune responses. These findings not only deepen our understanding of host-virus interactions but also suggest PARP12 as a potential therapeutic target for influenza and other viral infections.
The study was led by corresponding authors Academician Junying Yuan and Associate Researcher Heling Pan from the Shanghai Institute of Organic Chemistry, CAS, with Dr. Xin Huang as first author. Researcher Xiaozhen Liang and graduate student Fangxia Li, from the Shanghai Institute of Immunity and Infection, CAS, provided valuable assistance to this work. This research was supported by grants from the National Natural Science Foundation of China, Chinese Academy of Sciences, and Shanghai Municipal Science and Technology Commission.
Yuan's group discovered PARP12 mono-ADP-ribosylates RIPK1/3, promoting necroptosis while inhibiting apoptosis. PARP12 knockout mice showed reduced influenza lethality and lung damage, revealing PARP12 as a cell death-immune checkpoint and potential antiviral target. (PNAS 2025)
Keyword: PARP12, MARylation, RIPK1, IFN pathway, IAV infection
Junying Yuan/Heling Pan
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Haike Road 100 Shanghai 201210 China
Tel: 0086-21-68582263
Email: junying_yuan@sioc.ac.cn; panheling@sioc.ac.cn
附件下载:
