Nature Communications | Liu Cong and Collaborators Discover That O-Glycopeptides Remodel Aβ Aggregation and Attenuate Its Neurotoxicity
Date:2025-07-14
Amyloid-β (Aβ) aggregation is considered one of the central pathogenic mechanisms underlying Alzheimer’s disease (AD). Elucidating the structural transitions that occur during pathological protein phase separation and aggregation, as well as the factors that regulate these processes, remains a key focus in neurodegeneration research. Previous studies have shown that certain bioactive molecules, nanostructures, or peptides can alter the aggregation pathway of amyloid proteins such as Aβ, thereby influencing fibril conformation and toxicity. However, most existing strategies focus on inhibiting aggregation or blocking the formation of toxic oligomers, whereas relatively few have sought to deliberately steer pathological proteins toward forming low-toxicity, stable aggregates.
Glycosylation—a widespread post-translational modification in mammalian proteins—plays a critical role in stabilizing protein structures and modulating molecular interactions, and has therefore attracted growing interest. Understanding how glycopeptides regulate amyloid protein aggregation holds significant scientific and therapeutic potential.
Recently, a collaborative team led by Prof. Cong Liu at the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Prof. Suwei Dong at the State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, and Prof. Yuan Liu and Prof. Chu Wang at the College of Chemistry and Molecular Engineering, Peking University, published a research article in Nature Communications titled “An O-glycopeptide participates in the formation of distinct Aβ42 fibril structures and attenuates Aβ42 neurotoxicity”.
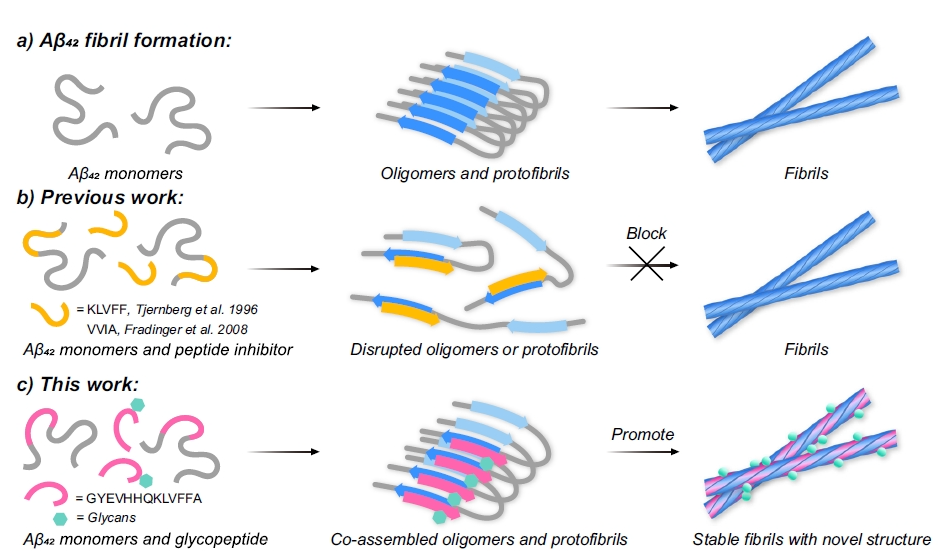
Figure 1. Self-assembly of Aβ42 and representative modulators of the assembly process
This study reports that a tyrosine O-glycosylated peptide with a specific β-N-acetylgalactosamine (β-GalNAc) modification can co-assemble with Aβ42, accelerating the formation of novel amyloid fibril structures that reduce toxic oligomer accumulation and alleviate neurotoxicity. The team designed a series of O-glycosylated peptides based on the Aβ aggregation core fragment (Gly9–Ala21), incorporating diverse monosaccharide units and glycosidic linkages, to evaluate their effects on Aβ42 aggregation. Screening identified β-GalNAc–modified Aβ9–21 (4b) as a strong promoter of Aβ42 fibril formation, while reducing the build-up of toxic oligomers (Figure 1), indicating a shift toward low-toxicity aggregates.
To investigate the molecular basis of this effect, the researchers used cryo-electron microscopy to determine the structures of Aβ42–4b fibrils (Figure 2). These fibrils displayed well-defined helical parameters and β-sheet cores, with increased hydrophobic interactions and tighter molecular packing compared to Aβ42 fibrils alone. Structural modeling with Rosetta and molecular dynamics simulations revealed that β-GalNAc moieties form stable hydrogen-bond networks between the amide N–H of one glycan and the C4 hydroxyl group of a β-GalNAc on an adjacent layer, thereby stabilizing the fibril architecture.
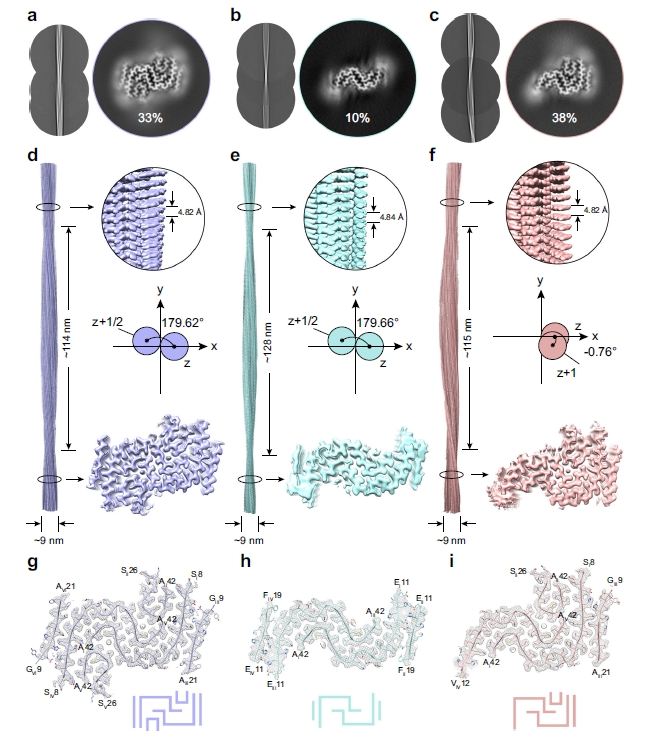
Figure 2. Cryo-EM structure determination of three major polymorphs of Aβ42-4b fibrils
Functionally, 4b significantly reduced Aβ42-induced cytotoxicity in neuronal cell models and improved cognitive performance and neuropathology in AD transgenic mice. These findings not only confirm the neuroprotective potential of glycopeptides in vitro and in vivo but also provide proof-of-concept for an “aggregation promotion as detoxification” strategy in amyloid-related diseases.
Overall, this work integrates glycopeptide design and synthesis, high-resolution structural analysis, molecular simulations, and biological validation to reveal the structural basis and mechanism by which O-glycopeptides modulate Aβ42 aggregation and toxicity. It proposes a novel concept that glycosylated peptides can direct Aβ toward forming more stable, less toxic fibrillar aggregates, and highlights the potential of glycopeptides as molecular tools to reprogram amyloid aggregation pathways.
Prof. Suwei Dong (Peking University), Prof. Cong Liu (Shanghai Institute of Organic Chemistry, CAS), and Prof. Yuan Liu (Peking University) are the co-corresponding authors. The first authors are Qi-jia Wei and Dangliang Liu (School of Pharmaceutical Sciences, Peking University, Class of 2022), Wencheng Xia (Interdisciplinary Research Center on Biology and Chemistry, CAS, Class of 2025), and Fengzhang Wang (College of Chemistry, Peking University, Class of 2025). The work was supported by the National Natural Science Foundation of China, Shanghai Municipal Science and Technology Commission, Shanghai Shangsi Institute for Natural Sciences, and the Chinese Academy of Sciences.
附件下载:
