Molecular Cell | Yixiao Zhang’s Team Uncovers the Regulatory Mechanism of XPR1, a Key Protein in Brain Calcification
Date:2025-08-26
Phosphorus is indispensable for human life, participating in nucleic acid synthesis, energy metabolism, signal transduction, and bone development. While phosphate uptake is essential, excessive accumulation leads to complexation with calcium, magnesium, and iron, causing cellular and tissue toxicity. To maintain homeostasis, an efflux mechanism is required. XPR1 is the only known phosphate exporter in humans, and its dysfunction has been linked to disorders such as brain calcification, thrombosis, and cancer. In primary basal ganglia calcification, loss of XPR1 function disrupts phosphate efflux, promoting calcium–phosphate deposition and neurological impairment.
XPR1 activity is intracellularly regulated by the inositol pyrophosphate InsP8, a molecular indicator of phosphate concentration. In parallel, the neuronal scaffold protein KIDINS220 forms a complex with XPR1 to modulate its function. However, the molecular mechanisms underlying the actions of InsP8 and KIDINS220 remain poorly understood, limiting insight into the pathogenesis of XPR1-related diseases.
A recent study led by Prof. Yixiao Zhang at the Interdisciplinary Research Center on Biology and Chemistry, and State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, together with Prof. Ben Corry (Australian National University), Prof. Yadong Sun (ShanghaiTech University), and Prof. Stephen Shears (National Institute of Environmental Health Sciences, USA), addresses this gap. Their article, “KIDINS220 and InsP8 safeguard the stepwise regulation of phosphate exporter XPR1”, published in Molecular Cell, combines cryo-electron microscopy, functional assays, and computational simulations to uncover two previously unrecognized regulatory mechanisms: a “key-to-locks” activation mode and a “knock–kiss–kick” efflux process. The work clarifies the molecular basis of pathogenic XPR1 mutations and delineates evolutionary conservation and divergence across XPR1 homologs.


Figure 1. Structure of the XPR1–KIDINS220 complex and the full conformational change process of XPR1 activation by InsP8
“Key-to-locks” Activation
High-resolution cryo-EM structures of XPR1 bound to KIDINS220 and to InsP8 revealed multiple activation states. KIDINS220 stabilizes XPR1 in a closed conformation by restricting movements of the SPX domain. Within this arrangement, the α1 helix acts as a structural hub, reinforcing the locked state. Binding of InsP8 to the α1 helix lifts these restrictions, initiating activation.
Activation proceeds stepwise: the C-loop, acting as a “lid,” blocks the intracellular entry. InsP8 induces a 180° flip of the SPX domain, exposing an interface that interacts with the C-loop, thereby opening the entrance. Finally, outward movement of the TM9b helix enlarges the extracellular exit, enabling phosphate release. Collectively, activation requires sequential removal of restraints by KIDINS220, the α1 helix, the C-loop, and TM9b — a finely tuned “key-to-locks” regulatory mechanism.
“Knock–kiss–kick” Efflux
Structural and computational analyses further revealed that intracellular and extracellular gating of XPR1 is decoupled. Four distinct conformations were observed: closed/closed, open/closed, closed/open, and open/open. This uncoupled behavior is unlike classical transporters.
A narrow constriction site persists across all conformations, suggesting phosphate transport requires additional forces. Molecular dynamics simulations revealed a three-step efflux process: 1) Knock – A phosphate ion repels negatively charged residues at the constriction site, transiently opening the gate. 2) Kiss – The phosphate briefly occupies a positively charged pocket, stabilized yet destabilized by nearby polar and acidic residues. 3)Kick – Entry of the next phosphate ion displaces the bound one, driving efflux. This sequence constitutes a unique “knock–kiss–kick” model for phosphate transport through XPR1.
Pathogenic Mechanisms in PFBC
The study also provides mechanistic insights into primary familial brain calcification (PFBC). Two recurrent patient mutations, R459C and R570L, localize to critical gating regions. Structural analysis showed that R459C mimics the InsP8-bound active state with SPX flipped and the intracellular entrance open, whereas R570L stabilizes an outward-open conformation. Despite resembling activated states, both mutants disrupt the channel environment, impairing phosphate binding and efflux. These defects ultimately drive pathological calcium–phosphate deposition, offering a direct molecular explanation for PFBC.
Evolutionary Insights
Comparisons with homologs in other species revealed both conservation and divergence. PXo from fruit flies maintains a constitutively open extracellular exit, and neither InsP6 nor InsP8 stabilizes its SPX domain, indicating distinct regulatory logic. In contrast, PHO1 from plants forms asymmetric dimers, though the functional relevance of this asymmetry requires further study. Together, these findings highlight both the conservation and diversification of phosphate efflux mechanisms during evolution.
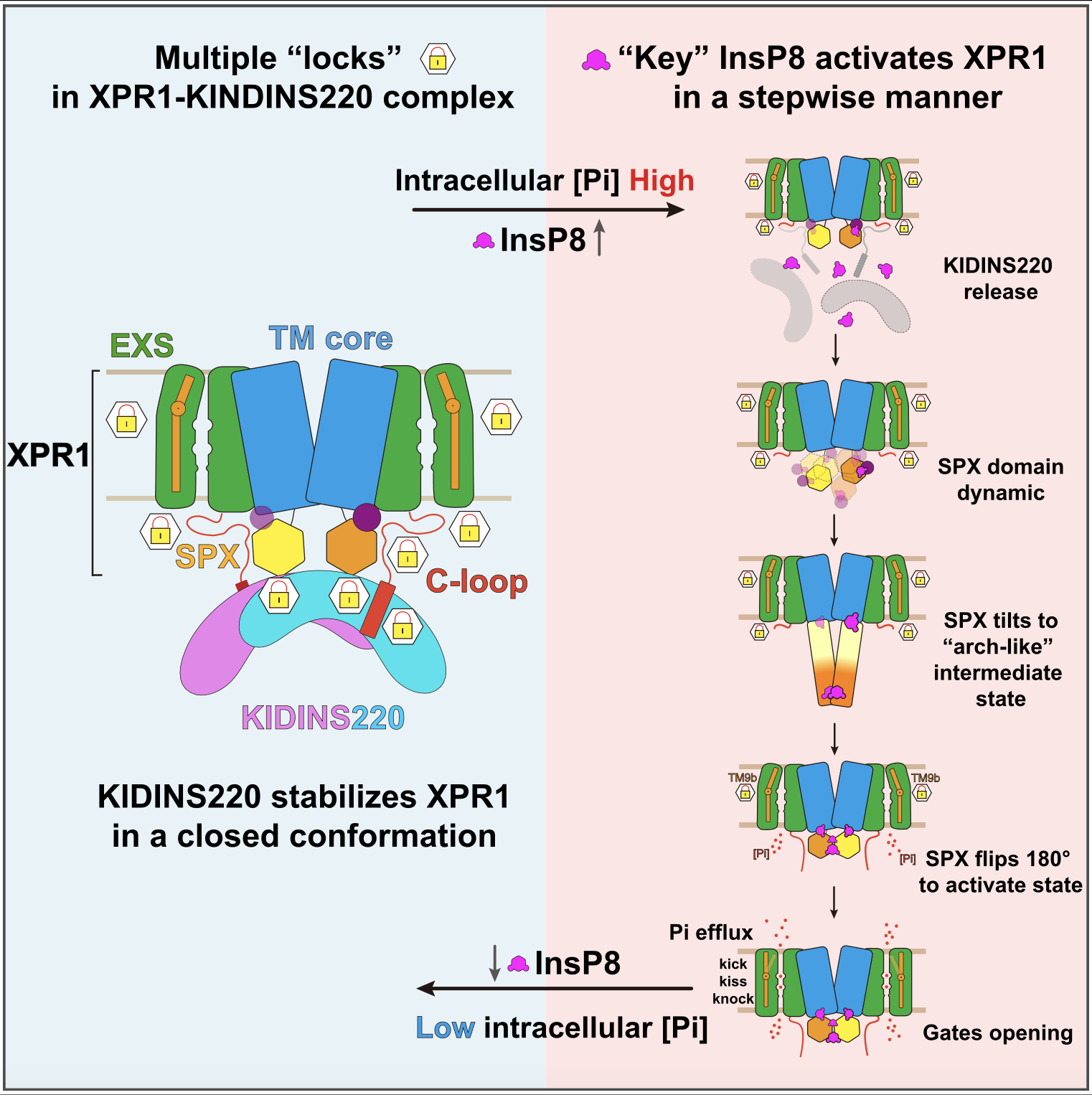
Figure 2. Model of XPR1 phosphate transport activity regulated by KIDINS220 and InsP8
This work establishes two conceptual advances: The “key-to-locks” model, in which InsP8 sequentially removes restraints imposed by KIDINS220 and intrinsic elements of XPR1. The “knock–kiss–kick” model, describing a phosphate efflux mechanism distinct from classical transporter paradigms. These discoveries provide a molecular framework for understanding phosphate homeostasis and lay the foundation for exploring therapeutic interventions in XPR1-related diseases such as brain calcification and cancer.
Dr. Xiaojie Wang and Dr. Zhongjian Bai (at the Interdisciplinary Research Center on Biology and Chemistry, and State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences) and Dr. Ciara Wallis (Australian National University) are co-first authors. Prof. Yixiao Zhang, Professor Ben Corry, Professor Yadong Sun, and Professor Stephen Shears are corresponding authors. Professor Tian Yang (Second Military Medical University), Professor Mingguang Lei (Hangzhou Normal University), and other colleagues made significant contributions.
Original article link: https://doi.org/10.1016/j.molcel.2025.08.003
附件下载:
